Reaction Documentation for
The Reactions of RO2 with NO
Available rate coefficients for the reactions of RO2 with NO have been reviewed by Lightfoot et al. (1992), Wallington et al. (1992; 1997), Atkinson et al. (1999) and Tyndall et al. (2001), with recommendations made in a number of cases. Where applicable, the kinetic data applied to these reactions in the MCM are consistent with these recommendations. For the vast majority of the ca. 900 RO2 radicals, however, kinetic data are unavailable, and the assigned rate coefficients are based on two generic expressions. For acyl peroxy radicals a value of
is used, based on the temperature-dependent rate coefficient reported for CH3C(O)O2 by Villalta and Howard (1996). For other classes of peroxy radical, rate coefficients are defined by the experssion
which is the product of the rate coefficient and an efficiency factor, ƒ. The rate coefficient is based on recommended values for CH3O2 and C2H5O2 (Atkinson et al., 1999), and the observations of Eberhard et al. (1997) and Eberhard and Howard (1996; 1997) for C3-C5 alkyl peroxy radicals. A unity value of ƒ is thus applied to alkyl peroxy radicals in general. In contrast to the treatment of alkyl peroxy radicals in our previous protocol (Jenkin et al., 1997), therefore, there is no dependence of the rate coefficient on peroxy radical size. A unity value of ƒ is also applied to the majority of other peroxy radicals with the exception of α-chlorinated peroxy radicals and β-chlorinated peroxy radicals, for which respective values of 2.2 and 1.6 are used, to account for the consistently larger reported rate coefficients for a number of C1 and C2 halogenated RO2, as described previously (Jenkin et al., 1997).
The following two channels are considered for the reactions of RO2 with NO:
| RO2 + NO | → | RO + NO2 | (11a) |
| → | RONO2 | (11b) |
The assigned branching ratios for the nitrate-forming channels, k11b/(k11a + k11b), are based on experimental measurements, where possible (Carter and Atkinson 1989; Lightfoot et al., 1992).
Where data are not available, ratios for other primary, secondary and tertiary alkyl peroxy radicals are calculated using the expression recommended originally by Carter and Atkinson (1989) and subsequently by Atkinson (1990, 1994). On the basis of available data for peroxy radicals formed from propene, methyl propene and cis-2-butene (Shepson et al., 1985; Lightfoot et al., 1992; Muthuramu et al., 1993), ratios for β-hydroxy RO2, for which experimental data are not available, are taken to be 0.5 of the values for the corresponding unsubstituted alkyl RO2.
With the exception of δ-hydroxy RO2 radicals, branching ratios for other RO2 possessing functional groups remote from the peroxy radical centre are also calculated using the expression of Carter and Atkinson (1989) for alkyl peroxy radicals of the same carbon number. Ratios for δ-hydroxy RO2 (i.e. formed following 1,5 isomerisation reactions of oxy radicals as described below) have long been suspected to be somewhat lower than for the unsubstituted counterparts (Carter and Atkinson (1985); Carter 1995), and nitrate formation has often been assumed not to occur in these cases. However, δ-hydroxy alkyl nitrates have recently been detected as products of the oxidation of hexane (Eberhard et al. (1995)). The tentative yields in that study, and the recently observed chain formation of NO2 during the oxidation of a series of C1-C8 alkanes (Hoffmann et al., 1995) may be interpreted in terms of the formation of the organic nitrate being ca. 40% as efficient for δ-hydroxy RO2 compared with the corresponding alkyl peroxy radicals (i.e. the same carbon skeleton), and this is assumed in the present work.
For acyl and α-carbonyl RO2 radicals, the ratio is taken as zero, on the basis of observations for CH3C(O)O2 and CH3C(O)CH2O2 (Lightfoot et al., 1992). For simplicity, and in the absence of definitive product data, the ratios for the β-nitro-oxy peroxy radicals are assumed to be zero.
On the basis of data presented by Lightfoot et al., 1992, values of k11b/(k11a + k11b), for the C5 alkyl type peroxy radicals derived from the OH-initiated oxidation of isoprene are all taken to be 0.10. Ratios for the similar C4 peroxy radicals formed from 1,3-butadiene are assumed to be 0.065, on the basis of the relative ratios calculated for C4 and C5 alkyl peroxy radicals (Carter and Atkinson (1989)).
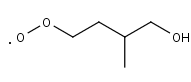 C52O2
C52O2
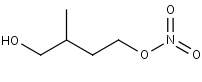 C52NO3
C52NO3