Reaction Documentation for
Initiation by photolysis
Photolysis reactions areconsidered for simple carbonyl compounds, such as aldehydes and ketones which are both emitted into the troposphere and formed as degradation products, and also for many other complex carbonyl compounds, hydroperoxides and organic nitrates which are generated as degradation products. Certain classes of compound containing carbonyl groups (e.g. carboxylic acids and esters) do not absorb significantly at wavelengths above 290 nm (Calvert and Pitts, (1966)), and photolysis reactions are therefore not considered for these species.
As described in more detail in Jenkin et al., (1997), the methodology used in the MCM involves assigning photolysis parameters to a core number of reactions (shown in Table 1) for which absorption cross section and quantum yield data are available. Some of these parameters are also used to define the photolysis rates of a much larger number of related species, for which the required information is not available (e.g. C2H5C(O)CH3 is used as a surrogate for aliphatic ketones. This procedure is described fully in Jenkin et al., (1997).
For much of the work performed with the MCM (see models in the "downloads" section ), photolysis rates as a function of solar zenith angle have been determined from the available data for the core reactions using a two stream isotropic scattering model described in Hayman, (1997). Calculations were performed for clear sky conditions at an altitude of 0.5 km on 1 July at a latitude of 45o N. In each case, variation of photolysis rate with solar zenith angle can be described well by an expression of the following form:
j = l(cos χ)mexp(–n.sec χ)
by optimising the values of the three parameters, l, m and n (see discussion in Jenkin et al., (1997)). The optimised parameters for all the core photolysis reactions are presented in Table 1.
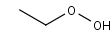 C2H5OOH
C2H5OOH
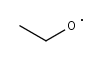 C2H5O
C2H5O